Complete And Balance The Following Equation Al Cl2. Once you know how many of. On left side there are 4 hydrogen atoms and 2 hydrogen atoms on right.put a coefficient 2 in front of the
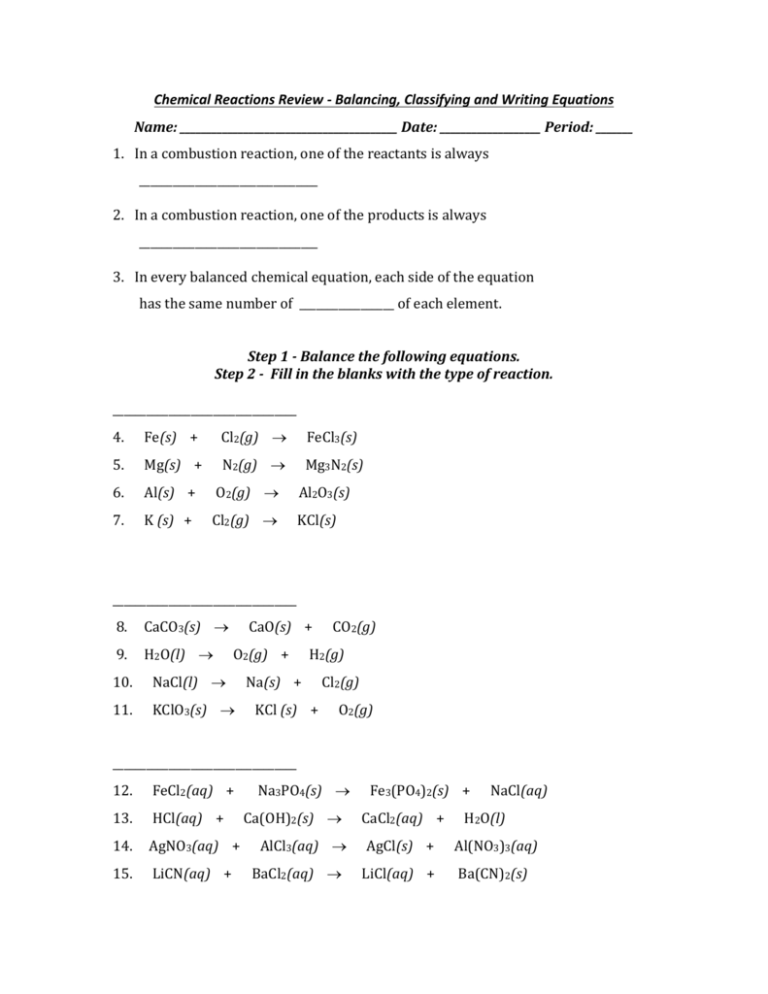
Balance the following equations, and indicate whether they are combination, decomposition, or combustion reactions.al(s) + cl2(g) → alcl3(s) Create a system of equations, one per element al: In order to balance al + cl2 → alcl3 you'll need to be sure to count all of aland cl atoms on each side of the chemical equation.
Write A Balanced Equation To Represent This Reaction.
Since there are two chlorine atoms on the left side, there needs to be two chlorine atoms on the right side. Balanced with 1 c on both side. Complete and balance the following equation:
Label Each Compound With A Variable A Al B Cl 2 = C Alcl 3 2.
Which of the following equations correctly describes the relationship between the rate at which no2 and cl2 are consumed in the following reaction? Al (s)+6hcl (aq)→alcl3 (aq)+3h2 (g) here are now 6 cl on the reactant side and 3 on the product side. Al(s) + (put only numbers in the spaces to balance even if it's 1) then complete the following table giving the moles, number of molecules, and total mass for each substance in the reaction.
Once You Know How Many Of.
By means of a stoichiometry, 1 mol of manganese dioxide reacts with 4 mol of hcl (hydrochloric acid) to form 1 mol manganese chloride 1 mol of chloride & 2 mol of water. Now there are two sodium and two chlorine atoms on the right side. Predict the product of the following reaction with a chemical equation calculator.
How To Balance The Equation Na (S)+Cl2(G) → Nacl.
For example, in the redox reaction of na and cl 2:. Al cl2 = alcl3 1. A) zn + h2so4 → znso4 + h2 b) cr + pbcl2 → crcl2 + pb c) ag + hcl → n.r.
Complete And Balance The Following Chemical Equation:
The original equation is na + cl = nacl. Chemical equation balancer helps you complete the process digitally. D) 2 al + 3 cuso4 → al2(so4)3 + 3 cu e) 2 li + 2 h2o → 2.